To understand the relationship between Kp and Kc, let us first understand these individually.
Kp is the equilibrium constant determined from a reaction equation’s partial pressures. It’s a mathematical expression for the dynamics between product and reactant pressures. Although it connects the pressures, it is a unitless number.
Concerning concentrations represented in molarity, Kc is the equilibrium constant.
Let us understand the relationship between Kp and Kc through an equation.
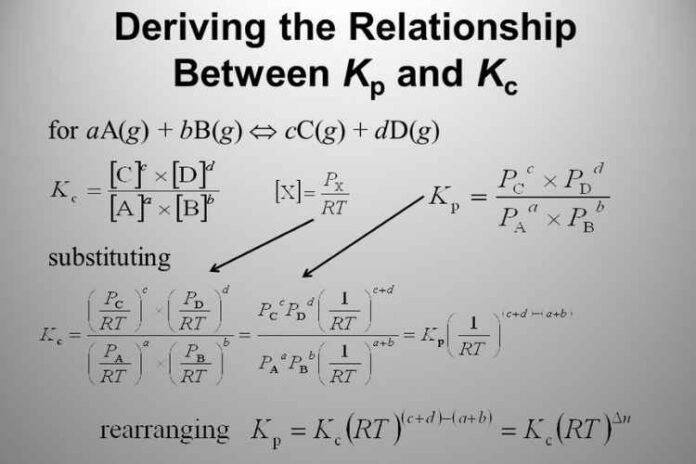
aA bB ⇋cC dD
(Where, a mole of reactant A, b mole of reactant B, c mole of product C, d mole of product D; a,b,c, and d are the Stoichiometric coefficients of reactants A, B and products C, D.)
The equilibrium constant in terms of concentration– (Equation 1)
(Concentration of C to the power c multiplied by the concentration of D to the power d, wholly divided by the concentration of A to the power a multiplied by the concentration of B to the power b)
Kc = [C]c [D]d [A] = molarity of A ([ ] = concentration)
[A]a [B]b = (Number of moles)
Volume
C = Molar concentration of product ‘C’
D = Molar concentration of product ‘D’
A = Molar concentration of product ‘A’
B = Molar concentration of product ‘B’
The equilibrium constant in terms of partial pressure-(Equation 2)
(Partial pressure of C to the power c multiplied by partial pressure of D to the power d, whole divided by partial pressure of A to the power a multiplied by partial pressure of B to the power b)
Kp = [PC]c [PD]d P = partial pressure
[PA]a [PB]b
PC = Partial pressure of product ‘C’
PD = Partial pressure of product ‘D’
PA = Partial pressure of product ‘A’
PB = Partial pressure of product ‘B’
Let us consider an ideal gas equation to derive the relationship.
Each of these ideal gas molecules behaves in the same way. As a result, for each of them,
PV = nRT
P = Pressure of the ideal gas
V= Volume of the ideal gas
n = number of moles
R = Universal gas content
T = Temperature
On rearranging the values, we get,
P = nRT
V
P = [ ] RT ( n/V = [ ])
(n = number of moles, V = volume, [ ] = concentration)
So, we can calculate partial pressures as:
PA = A RT
PB = B RT
PC = C RT
PD = D RT
Applying this to Equation 2,
Kp = ([C]RT)c ([D]RT)d
([A]RT)a ([B]RT)b
= KcRT(c d)-(a b) {(c d) – number of moles = np }
{(a b) – number of moles = nr }
Hence, (c d) – (a b) = np – nr = Δng {Δng = Difference in number of gaseous moles; (Number of gaseous moles in product side – Number of gaseous moles in reactant side)
= KcRTΔng
Thus, we get the relation between Kp and Kc as:
Kp = KcRTΔng
The Kp and Kc relationship will alter when the number of moles of gas molecules changes.
Case 1:
If the change in the number of moles gas molecules in the equation is 0
i.e., Δng = 0, then,
Kp = Kc
Case 2:
If the change in the number of moles gas molecules in the equation is positive
i.e., Δng > 0, then,
Kp > Kc
Case 3:
If the change in the number of moles gas molecules in the equation is negative
i.e., Δng < 0, then,
Kp < Kc
Conclusion
So did you understand the equilibrium concept or not? If you still have doubts, read further on the topic and know more.
Frequently Asked Questions (FAQs)
Q1. How are Kp and Kc related?
Ans. Kp and Kc are the gaseous mixture equilibrium constants in a reversible reaction, and they are directly proportional to each other, as shown by the equation Kp = KC(RT)ng.
Q2. Why is the temperature the only factor that affects KP and KC?
Ans. The temperature has an impact on Kc. The value of Kc will change when ‘T’ changes and this value will scale depending on the type of reaction (endothermic/exothermic).
Q3. Why are Kp and Kc different?
Ans. The equilibrium constants of gaseous mixtures are Kc and Kp. However, Kc is determined by molar concentrations, whereas Kp is defined by the partial pressures of the gases inside a closed system.
Q4. What can we deduce from the equilibrium constant?
Ans. The magnitude of the equilibrium constant, K, determines how far a reaction will progress: If K is a significant number, it indicates that the products’ equilibrium concentration is high. If K is a small number, it indicates that the reactants’ equilibrium concentration is high.
Q5. Is it always the case that KC equals KP?
Ans. Δn = moles of gaseous products – moles of gaseous reactants. Note that Kc = Kp when both sides have the same number of gas molecules.
Q6. Why aren’t KP and KC the same?
Ans. When Δn = 0, Kp equals Kc. This is true when the number of moles of gaseous products in the balanced chemical equation equals the number of moles of gaseous reactants. Kp can be less than Kc (for Δn < 0) or greater than Kc (for Δn > 0).
Q7. What is the equilibrium difference between KC and KP?
Ans. When using concentrations, Kc is the equilibrium constant. However, when using partial pressures, Kp is the equilibrium constant.
Q8. Why does temperature affect the value of KC?
Ans. When a reaction is exothermic, raising the temperature lowers Kc and vice versa. This is because raising the temperature causes the equilibrium to shift backwards (in the endothermic direction), causing the concentration of reactants to rise while the concentration of products falls.
Q9. Is the Kp/Kc relationship valid only for a gaseous mixture?
Ans. No, it is not. The relationship between Kp and Kc holds for all reversible processes, such as solid to gas, gas to solid, and so on.
Q10. Under what circumstances can a reaction’s KC and KP be equal?
Ans. For Kc and Kp to be equal, the volume of the reactants and products must be constant at equilibrium, that is, the volume of the reactants must be equal to the volume of the products on the product’s side.